Patient-Like Molecular Controls for Infectious Disease Testing
Exact Diagnostics—A Comprehensive Line of Highly-Characterized Molecular Controls & Panels
Partnering with You to Improve Patient Care
We understand that your work in molecular diagnostics helps enable powerful insights and new therapies. Bio-Rad is a recognized leader in quality control and data management. With our comprehensive line of molecular standards and controls, we can help you with your goal of providing critical and accurate diagnostic information. Together, we can advance the science of molecular diagnostics to drive efficiency, confidence, and better patient care.
Talk with a Specialist
Your molecular lab
Your molecular lab is enabling more powerful, personalized, and preventative therapies. Bio-Rad has the quality control products and expertise to help ensure the accuracy and value of your critical lab findings.

Bio-Rad quality controls
With Bio-Rad, you have a long-term QC partner with the resources, technology, and experience to help you elevate your understanding, bolster your progress, and empower groundbreaking work.

Improved performance for better patient care
Together, we can advance the science of molecular diagnostics to drive efficiency, confidence, and better patient care.
Setting a new standard in molecular lab performance. Together.
Bringing 60 years of quality control expertise to molecular labs

Bio-Rad has been a leader in quality control for more than 60 years and we are bringing this expertise to molecular labs with:
- A comprehensive portfolio of molecular standards and controls across key diagnostic areas
- Integration with Unity QC Data Management, which helps laboratory personnel connect and compare their data with labs worldwide.
- Highest level of customer service and technical support
Benefits of Exact Diagnostics Molecular Quality Controls and Verification Panels
Measured with ddPCR for the absolute and unbiased quantification or calibration of verification panels, controls, and standards
Verification panels are traceable to available WHO international standards
Verification panels manufactured according to ISO 17511 for the highest quality and consistency
High-quality matrices help ensure coherent QC results
Lot-to-lot reproducibility helps maintain consistency of QC and quantitative test results
Longer shelf lives help save time on crossover testing and money
Comprehensive Portfolio of Molecular Diagnostic Standards and Controls
The Exact Diagnostics line of molecular standards and controls can provide high-quality QC solutions for key diagnostic areas. These products feature unsurpassed quality, characterization, and consistency for which Bio-Rad is known. Our growing portfolio includes innovation in syndromic panels and other molecular applications. Our key diagnostic areas include transplantation, virology, sexually-transmitted infections, microbiology, hospital-associated infections, respiratory illness, and tick-borne diseases.
Meningitis and Encephalitis
Quality controls and verification panels to monitor the presence and establish points of reference for the measurement of pathogens common in meningitis and encephalitis testing.
Microbiology
Quality controls to monitor the presence of pathogens common in microbiology such as C. difficile, MRSA, and TV/MG
Respiratory
Multi-analyte quality controls to monitor the presence and establish points of reference for the measurement of common respiratory pathogens including SARS-CoV-2
Transplant
Quality controls and verification panels to monitor the presence and establish points of reference for the measurement of pathogens common in meningitis and encephalitis testing
Tick-Borne
Quality controls to monitor the presence of common vector-borne pathogens such as Anaplasma, Babesia, Bartonella, Borrelia, and Ehrlichia
Virology
Quality controls and verification panels to monitor the presence and establish points of reference for the measurement of common viruses such as HIV-1, HIV-2, HBV, HCV and Zika
Sexually Transmitted Infections
Quality controls for use with nucleic acid assays detecting common pathogens causing sexually transmitted infections (STI) such as CT/NG and HPV
Hospital Associated Infections
Quality Controls to monitor the presence of pathogens such as Enterovirus, which is common in Meningitis
Negative
Quality controls to monitor the absence of common pathogens found in Transplant, Vector-Borne, Meningitis and Encephalitis, Sexual Health and Hospital-Associated Infections
Experience the Bio-Rad Advantage
Integrated Unity software solutions
By partnering with Bio-Rad, your lab can access the powerful Unity QC data management, which provides access to our interlaboratory program. Assessment tools and reports can help your lab meet regulatory and compliance requirements, streamline your QC workflow and process, create standards for testing and benchmarking lab performance, and report results with confidence.
Highest level of customer service, partnership, and technical support
Bio-Rad can also support your lab with more than QC. We provide QC technical support, program support for both Unity and the QCNet portal, software and connectivity support, and specialized technical proficiency in the form of trained specialists.
Resources
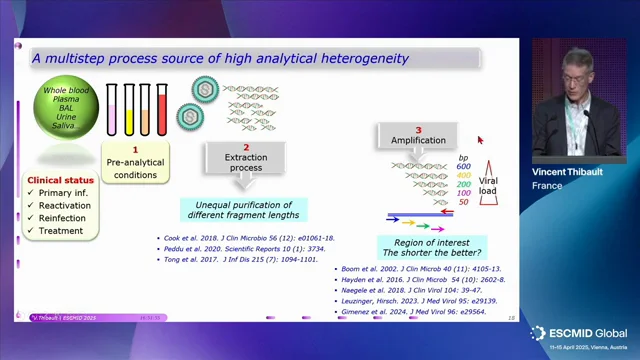
CMV Viral Load Monitoring in Transplant Patients
Explore the present and future of CMV viral load monitoring in transplant patients, covering quantification harmonization to new therapeutic strategies in this ESCMID Symposium session.

Optimizing GBS Quality Control in Lim Broth
Learn how swab immersion timing and matrix type impact Group B Streptococcus (GBS) detection in expectant mothers using a GBS LB assay. This study emphasizes the need for precise quality controls to ensure reliable PCR results, improving newborn outcomes.

GBS QC Poster at Clinical Virology Symposium (CVS)
Discover key findings from our CVS poster on optimizing Group B Streptococcus (GBS) quality control with a GBS LB assay. Explore how swab immersion timing and matrix type affect GBS detection accuracy, ensuring reliable PCR results for better newborn outcomes.

CMV DNA Quantification Variability Study
Published in the Journal of Clinical Microbiology, examine inter-laboratory variability in CMV DNA quantification across seven hospital labs, emphasizing the need for standardized calibrators to enhance accuracy in monitoring immunocompromised patients.

Viral Load QC Optimization Study
Explore our Journal of Clinical Microbiology paper on optimizing quality control for CMV, EBV, and HIV viral load assays. Using Unity Real Time, we assess performance and recommend QC rules to enhance accuracy in molecular diagnostics.